FDA Approves First Therapy for Menkes Disease
|
General Studies Paper II: Health |
Why in News?
The U.S. Food and Drug Administration (FDA) has approved Zycubo (copper histidinate) as the first and only therapy for Menkes disease. This subcutaneous treatment significantly improves survival in affected infants by delivering usable copper, offering new hope for children who previously had no approved options.
What is Menkes Disease?
- About: Menkes disease (also called Menkes syndrome or kinky hair disease) is a rare genetic disorder of copper metabolism caused by mutations in the ATP7A gene, which impairs the body’s ability to absorb and distribute copper. This leads to deficient copper levels in many organs.
- Causes: Menkes disease results from mutations in the ATP7A gene, which encodes a copper‑transporting P‑type ATPase protein essential for moving copper from the intestine into the bloodstream and into cells throughout the body. Many cases are inherited from carrier mothers, while about one‑third arise from spontaneous mutations.
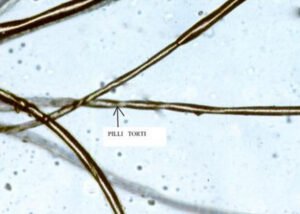
- Symptoms: Clinical signs usually appear between 2–3 months of age after a brief healthy period at birth. Common early symptoms include sparse, coarse, brittle, and light‑colored (kinky) hair, failure to thrive, developmental delays, seizures, hypotonia (weak muscles), low body temperature, and feeding difficulties. Severe neurological degeneration and intellectual impairment develop rapidly.
- First Case: Menkes disease was first described in 1962 by pediatrician John Hans Menkes, who recognized a unique syndrome of growth failure, developmental delay, and peculiar hair texture in affected infants. The defective copper transport due to ATP7A mutations was elucidated later with advances in molecular genetics.
- Severity: Menkes disease is severe and often lethal. Without early treatment, most affected children experience progressive neurological damage, failure to thrive, and death typically before age 3.
- Affected Parts of the Body: Due to widespread copper deficiency, multiple systems are involved:
- Brain and nervous system: progressive neurodegeneration, seizures, developmental delay.
- Hair and skin: fragile, kinky, depigmented hair; light skin coloring.
- Bones and connective tissues: osteoporosis, fractures, sagging skin.
- Blood vessels: fragile, tortuous arteries prone to rupture or blockage.
- Metabolism: impaired collagen formation and neurotransmitter synthesis due to inactive copper enzymes.
- Diagnosis: Diagnosis is suspected based on clinical features and confirmed through blood tests showing low serum copper and ceruloplasmin, microscopic hair analysis, and genetic testing for ATP7A mutations.
- Treatment: There is no definitive cure for Menkes disease, but early intervention with copper supplementation, especially copper histidinate injections can improve copper levels and enzyme function. Now, the FDA approved Zycubo (copper histidinate) as the first specific therapy, offering hope for better outcomes.
What is Zycubo (Copper Histidinate)?
- Zycubo is a medicinal formulation of copper histidinate. It represents a targeted, copper-replacement therapy designed to deliver bioavailable copper directly into the body, addressing the rare genetic disorder.
- The active ingredient in Zycubo is copper histidinate, a complex of elemental copper bound to the amino acid histidine.
- The formulation is supplied as a sterile lyophilized powder that is reconstituted and administered by subcutaneous injection (under the skin).
- Zycubo is administered by subcutaneous injection. In infants under one year of age, it is typically given twice daily, while older children and adolescents often receive it once daily.
- Zycubo is indicated exclusively for the treatment of Menkes disease in children and adolescents. It is not approved for related ATP7A-associated conditions such as Occipital Horn Syndrome.
- The therapeutic benefit of Zycubo was established in open‑label, single‑arm clinical trials. In these trials, children who began Zycubo within four weeks of birth showed a 78% reduction in the risk of death and a median overall survival of 17.1 months compared to 16.1 months in untreated historic controls.
Zycubo Therapy Mechanism
- Bypassing the Genetic Defect: Zycubo (copper histidinate) works by bypassing the defective ATP7A transporter. Because patients cannot absorb copper normally due to this genetic mutation, Zycubo is formulated as a subcutaneous injectable, allowing copper to enter circulation directly rather than through the digestive tract — addressing the core biochemical barrier of the disease.
- Delivering Bioavailable Copper Systemically: Once injected, Zycubo rapidly increases systemic copper levels in the blood. The copper in Zycubo is bound to histidine, forming a stable complex that cells can take up more readily. This increases the availability of biologically active copper ions throughout the body, helping to overcome the severe deficiency caused by the inability to absorb copper naturally.
- Restoring Copper-Dependent Enzyme Function: With copper now present in the bloodstream and delivered to tissues, essential copper-dependent enzymes begin to function more effectively. These include enzymes involved in neurodevelopment, connective tissue formation, energy metabolism, and antioxidant defense. By restoring copper to these pathways, Zycubo helps mitigate the neurological degeneration, connective tissue abnormalities.
|
Biochemical Role of Copper
|